As the global population increases and the diets of people around the world include more protein, potash production is becoming increasingly important. Primarily used to produce fertiliser, potash refers to a broad range of compounds bearing potassium (K). As one of the “Big 3” essential nutrients in commercial fertilisers, NPK – Nitrogen, Phosphorus & Potassium[1], potassium is vital to crop health and a key component of the global food supply chain.
 Global Potash Production
Global Potash Production
Potash is primarily produced industrially as potassium chloride (KCl), also known as muriate of potash (MOP). Recent years have also seen an increase in production of potassium sulphate (K2SO4) or sulphate of potash (SOP) as a premium alternative.
Various recovery and processing methods are employed for potash. Potash ore is typically found in ancient, evaporated oceans, with the most significant deposits found in the Canadian province of Saskatchewan. Potash ore is extracted via surface, underground and cavern solution mining. Pond-evaporated seawater, such as from the Dead Sea, may also be employed as a potash feedstock.
Potash refining generally involves solids separation, brine concentration and evaporative crystallisation. In 2019, global production of potash was 66 MT (K2O equivalent), with the majority produced in Canada, Russia, Belarus and China[2].
 Process Modelling for the Potash Industry
Process Modelling for the Potash Industry
A good quality model of your operation is crucial for process optimisation, energy integration and quality control. A model built on robust principles can identify process deficiencies and bottlenecks while evaluating and ranking improvement opportunities.
Process models can be tied to financial gains through incorporation of raw material and operating costs, and commodity prices. Dynamic simulation may be used to size process equipment, evaluate downtime impacts and design control systems. Dynamic models may also be used for long-term scheduling of cavern solution mining or inventory management in evaporation ponds.
SysCAD is a leading simulation platform in the mining and minerals industries with significant capability in chemical thermodynamics, solids modelling, and mass and energy balances. SysCAD provides a suite of specialised features and unit operations required for rigorous modelling of your potash process.
SysCAD ships with a range of potash example projects related to the production of MOP and SOP. Other example projects also demonstrate various unit operations using particle size distribution, integration of thermodynamic calculations and other advanced features.
SysCAD Potash Add-On for Muriate of Potash
SysCAD offers a specialised Potash Add-On including a species model for accurately calculating MOP properties, built upon industry-standard solubility data from D’Ans (1952)[3]. This add-on allows for in-line calculation of potassium chloride (KCl) and sodium chloride (NaCl) solubilities in the presence of magnesium chloride (MgCl2) and low concentrations of calcium sulphate (CaSO4) and calcium chloride (CaCl2).
The Potash Add-On also includes a phase change subroutine (“PC evaluation block”) which uses these solubilities to automatically apply KCl and NaCl precipitation or dissolution within various generic process units throughout your circuit, including Ties, Tanks, Washers, Evaporators and Flash Tanks.
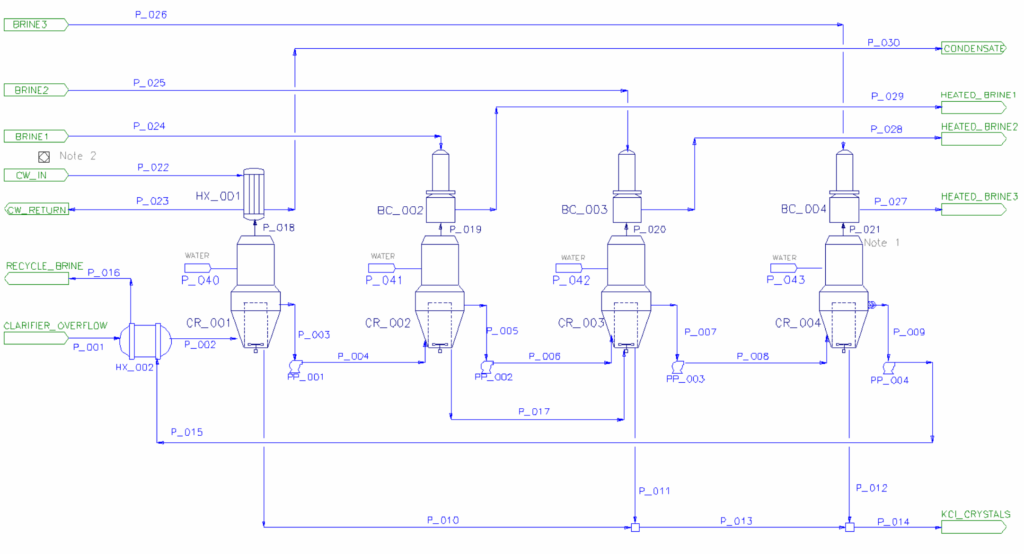 SysCAD Evaporator unit models use solubility equilibrium calculations to determine production from a crystallising evaporator. Combined with heat exchangers, compressors and condensers, the evaporator models integrate with the SysCAD flash train macro to detect steam consumers and producers, automatically calculating pressure distribution in the system. Importantly, this provides a mechanical steam balance for the process, an essential component to accurately model the circuit.
SysCAD Evaporator unit models use solubility equilibrium calculations to determine production from a crystallising evaporator. Combined with heat exchangers, compressors and condensers, the evaporator models integrate with the SysCAD flash train macro to detect steam consumers and producers, automatically calculating pressure distribution in the system. Importantly, this provides a mechanical steam balance for the process, an essential component to accurately model the circuit.
Thermodynamic Calculation Engines
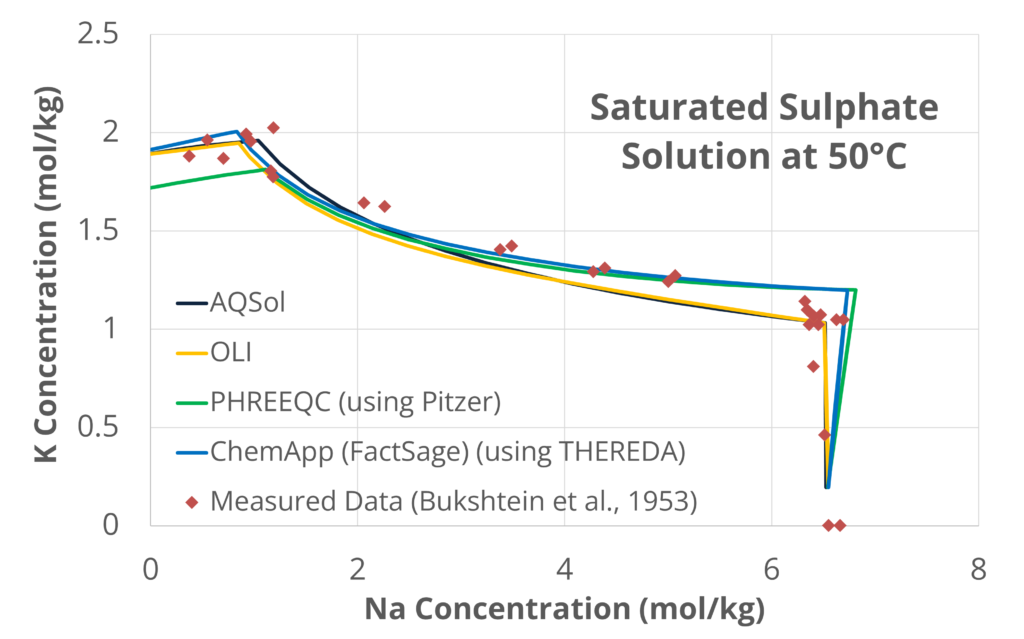
SysCAD offers seamless integration with various thermodynamic calculation engine (TCE) packages, namely AQSol, OLI, PHREEQC and ChemApp (FactSage). These packages can be used to perform rigorous phase equilibrium calculations on potash solutions over a wide range of temperatures and compositions.
Compared to the Potash Add-On, these packages can handle a wider range of species and predict the ingress of contaminants, but often require greater computational time. They are also applicable to both MOP and SOP processes. Solubilities from these TCEs may be applied within reaction units and dedicated evaporator models using the SysCAD flash train macro for steam and pressure balancing.
Compared with data from D’Ans (1933)[4] within a MOP system, the thermodynamic engines provide near-perfect correlation. The graph below shows validation against data from Bukshtein et al. (1953)[5] for a SOP system and the predicted solubilities[6] of potassium sulphate (K2SO4) and sodium sulphate (Na2SO4), in a saturated solution at 50°C.
 SysCAD Unit Operations and Features
SysCAD Unit Operations and Features
Alongside our potash and TCE offerings, SysCAD includes numerous generic unit operations of relevance to the potash industry, including:
- Grinding mills
- Flotation cells
- Thickeners and clarifiers
- Filters, screens and hydrocyclones
- Reaction vessels
- Evaporative crystallisers (optionally with internal recycle heat exchangers)
- Condensers
- Heat exchangers
SysCAD also features a Particle Size Distribution (PSD) Add-On, allowing for population balance modelling including particle breakage and growth throughout comminution, flotation, heavy media separation, tailings, and product crystallisation circuits. This multidimensional feature allows specification and calculation of individual mineral PSDs. Combined with powerful models of chemical solubility, product soluble losses in these circuits can be predicted and minimised.
SysCAD is the simulator of choice for the growing potash industry. With powerful capabilities in thermodynamics, and dedicated tools for potash systems, SysCAD is the foundation on which you can build the complete potash production process model.
Resources
- Online Documentation: Potash Models, Thermodynamic Calculation Engines
- We have a range of MOP and SOP Example Potash Projects available, including pond concentration, separation, and multi-stage crystallising evaporation. Please contact us to arrange a Trial Download.
References
- The Fertilizer Institute (2014) “Fertilizer 101: The Big 3 – Nitrogen, Phosphorus and Potassium” (Accessed 09 Dec 2021)
- Government of Canada (2021) “Potash Facts” (Accessed 09 Dec 2021)
- J. d’Ans (1952) “Die Bedeutung von Van Hoff’s Arbeiten Über Losungsgleichgewichte” Zeitschr. Electrochem
- J. d’Ans (1933) “Die Losungsgleichgewichte der Systeme der Salze Ozeanischer Salzablagerungen” Kali-Forschungsanstalt, Berlin, Germany
- V.M. Bukshtein, M.G. Valyashko, A.D. Pel’sh (Eds.) (1953) “Spravochnik po Rastvorimosti Solevykh System, Vols. I and II” Izd. Vses. Nauch.-Issled. Inst. Goz., Goskhimizdat., Moscow-Leningrad, 1270p
- H.C. Moog, F. Bok, C.M. Marquardt, V. Brendler (2015) “Implementation of a Thermodynamic Reference Database (THEREDA)”, Appl. Geochem. 55, 72-84p

